Macular Dystrophy Guide 2025: Symptoms & Treatments
Quick Answer: What is Macular Dystrophy?
Macular dystrophy represents a group of inherited genetic eye disorders causing progressive damage to the macula—the retina’s central region responsible for sharp, detailed vision. Unlike age-related macular degeneration, these conditions typically manifest in children and young adults through specific gene mutations. While macular dystrophy leads to central vision loss, peripheral vision usually remains intact. The most common forms include Stargardt disease, Best vitelliform macular dystrophy, and X-linked retinoschisis.
Introduction: Understanding Macular Dystrophy
Macular dystrophy represents a group of inherited eye disorders that specifically affect the macula—the small, central portion of the retina responsible for sharp, detailed vision. Unlike age-related macular degeneration, which typically affects older adults, macular dystrophy can strike children, teens, and young adults, fundamentally altering their vision and quality of life during critical developmental years.
These genetic conditions, collectively known as macular dystrophy, cause progressive damage to the light-sensitive cells in the macula, leading to central vision loss while usually preserving peripheral vision.
These genetic conditions cause progressive damage to the light-sensitive cells in the macula, leading to central vision loss while usually preserving peripheral vision. Macular dystrophies (MDs) consist of a heterogeneous group of disorders that are characterised by bilateral symmetrical central visual loss, making early diagnosis and comprehensive care essential for optimal patient outcomes.
Understanding macular dystrophy is crucial for patients, families, and healthcare providers, as advances in genetic testing over the last decade have led to improved knowledge of the underlying molecular basis. Modern research continues to unveil new therapeutic options for various types of macular dystrophy, with several breakthrough treatments emerging through clinical trials in 2025.
What Is Macular Dystrophy?
Macular dystrophy encompasses several inherited retinal diseases that share a common characteristic: progressive deterioration of the macula. This group of genetic disorders affects the specialized area of the retina containing the highest concentration of cone cells, which are responsible for central vision, color perception, and fine detail recognition.
Key Characteristics of Macular Dystrophy
Primary Clinical Features:
- Bilateral central visual impairment affecting both eyes symmetrically
- Preservation of peripheral vision in the majority of cases
- Progressive nature with variable rates of deterioration
- Autosomal dominant, autosomal recessive, or X-linked inheritance patterns
- Typical onset during childhood, adolescence, or early adulthood
Functional Impact: Macular dystrophy significantly impairs activities requiring central vision, including reading, writing, driving, recognizing faces, and performing detailed work. These limitations can profoundly affect educational achievement, career development, and social interactions, particularly given the young age of onset in most patients.
How Macular Dystrophy Differs from Age-Related Macular Degeneration
While both conditions affect the macula, macular dystrophy and age-related macular degeneration (AMD) represent distinct disease entities with important clinical differences:
Macular Dystrophy Characteristics:
- Monogenic inheritance with identifiable mutations in specific genes
- Early onset (typically before age 50, often in childhood or adolescence)
- Predictable progression patterns based on genotype
- Affects discrete retinal pathways according to the underlying genetic defect
- Multigenerational family involvement following Mendelian inheritance patterns
Age-Related Macular Degeneration:
- Multifactorial etiology involving age, environmental factors, and genetic predisposition
- Late onset (typically after age 60)
- Progression is influenced by modifiable risk factors, including smoking, diet, and cardiovascular health.
- Different therapeutic approaches targeting inflammation and neovascularization
Types of Macular Dystrophy
The classification of macular dystrophy includes several distinct genetic forms, each with unique characteristics, inheritance patterns, and progression rates. Understanding these different types of macular dystrophy helps patients and families navigate diagnosis, treatment options, and genetic counseling decisions.
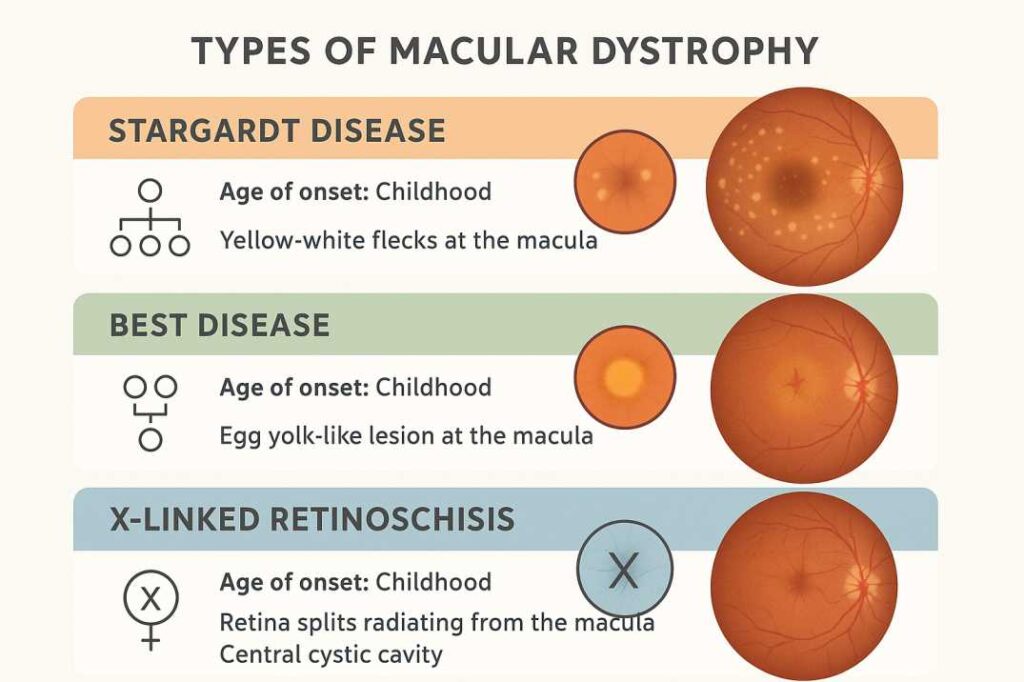
Stargardt Disease (STGD1)
Stargardt disease represents the most common inherited single-gene retinal disease, affecting approximately 1 in 10,000 individuals worldwide. This autosomal recessive condition results primarily from mutations in the ABCA4 gene, with over 1,000 variants identified to date. The discovery of these mutations has revolutionized the understanding of the disease’s pathophysiology and opened avenues for targeted therapeutic interventions.
Clinical Presentation: The disease typically manifests with bilateral central scotomas and distinctive retinal findings. Patients experience progressive central vision loss accompanied by characteristic yellow-white flecks visible on fundoscopic examination. The pathognomonic “silent choroid” appearance on fluorescein angiography, resulting from lipofuscin accumulation masking choroidal fluorescence, aids in diagnosis.
Pathophysiology: ABCA4 mutations impair the transport of vitamin A derivatives, leading to the accumulation of toxic bisretinoids, including lipofuscin and A2E. This accumulation causes progressive retinal pigment epithelium dysfunction and photoreceptor cell death, culminating in macular atrophy.
Best Vitelliform Macular Dystrophy
Best vitelliform macular dystrophy exhibits an estimated prevalence of 1 in 16,500 to 1 in 21,000 individuals, though recent population studies suggest higher rates in certain ethnic groups. This condition results from mutations in the BEST1 gene and follows an autosomal dominant inheritance pattern with variable expressivity and incomplete penetrance.
Distinctive Clinical Stages: The disease progresses through characteristic phases identifiable by fundoscopic appearance:
- Previtelliform Stage: Normal or near-normal fundus appearance with reduced electrooculogram responses
- Vitelliform Stage: Pathognomonic “egg-yolk” lesion formation representing lipofuscin accumulation
- Pseudohypopyon Stage: Partial resorption creating a layered appearance
- Vitelliruptive Stage: Lesion fragmentation resembling “scrambled eggs”
- Atrophic Stage: Progressive retinal pigment epithelium loss and chorioretinal atrophy
X-Linked Juvenile Retinoschisis
This condition almost exclusively affects males due to its X-linked inheritance pattern. XJR showing the classical “spoke-like” pattern in the macula is characteristic on examination.
Features:
- Retinal layer separation creates cystic spaces
- Potential for retinal detachment
- Gradual central vision deterioration
- Possible peripheral retinal involvement
Pattern Dystrophy
Pattern dystrophy presents with various retinal pigment patterns and can manifest in multiple forms, often developing in middle age with slower progression compared to other macular dystrophies.
Symptoms and Clinical Manifestations
Primary Visual Symptoms
Central Visual Disturbances: The hallmark of macular dystrophy involves progressive deterioration of central vision while peripheral visual fields remain largely intact. Patients typically report metamorphopsia (visual distortion), central scotomas (blind spots), and reduced visual acuity that cannot be corrected with conventional refractive correction.
Characteristic Presentations by Disease Stage:
Early Stage Manifestations:
- Subtle visual blurring during detailed tasks such as reading or needlework
- Difficulty discerning fine details in familiar faces
- Mild photophobia or increased sensitivity to bright illumination
- Possible nyctalopia (night vision difficulties) in certain subtypes
Progressive Stage Symptoms:
- Pronounced central scotomas interfere with daily activities
- Color vision deficiencies, particularly in blue-yellow discrimination
- Significant reading difficulties requiring magnification aids
- Challenges with depth perception and spatial orientation
Impact on Developmental Milestones
Given the typical onset during childhood and adolescence, macular dystrophy can significantly impact normal developmental processes. Educational achievement may suffer due to reading difficulties, while social development can be affected by challenges in facial recognition and participation in visually demanding activities.
Stages of Progression:
- Early Stage: Minimal symptoms, possible flecks visible only on examination
- Intermediate Stage: Noticeable central vision changes, difficulty with fine tasks
- Advanced Stage: Significant central vision loss, legal blindness possible
Impact on Daily Activities
Educational Challenges:
- Difficulty reading standard print
- Problems seeing blackboards or digital screens
- Challenges with detailed homework assignments
Social and Recreational Impact:
- Trouble recognizing friends at a distance
- Difficulty participating in sports requiring fine vision
- Challenges with hobbies requiring detailed work
Causes and Risk Factors
Genetic Foundations
All forms of macular dystrophy are caused by genetic mutations affecting retinal function, but the specific inheritance patterns and affected genes vary significantly between types. Understanding the genetic foundations of macular dystrophy is essential for accurate diagnosis, treatment planning, and family counseling.
Inheritance Patterns:
Autosomal Recessive (Stargardt Disease):
- Both parents must carry the mutated gene
- 25% chance of affected children when both parents are carriers
- Parents who each carry one mutated copy are called carriers and do not have symptoms
Autosomal Dominant (Best Disease):
- Only one parent needs the mutated gene
- 50% chance of inheritance from the affected parent
- Variable expression even within families
X-Linked (Juvenile Retinoschisis):
- Affects primarily males
- Transmitted through carrier mothers
- Affected fathers cannot pass the condition to their sons
Molecular Mechanisms
ABCA4 Gene Function: The ABCA4 protein transports potentially toxic substances that can damage photoreceptors. When this protein malfunctions, toxic byproducts accumulate, leading to:
- Lipofuscin buildup in retinal pigment epithelium
- Photoreceptor cell death
- Progressive macular atrophy
- Central vision loss
Other Genetic Pathways:
- BEST1 gene mutations affect ion channel function
- RS1 gene affects retinal structural integrity
- ELOVL4 and PRPH2 genes impact membrane composition
Diagnosis and Testing
Comprehensive Eye Examination
Initial Assessment: A thorough eye examination forms the foundation of macular dystrophy diagnosis. Eye care providers like those at Cannon EyeCare conduct comprehensive evaluations, including visual acuity testing at multiple distances, detailed visual field mapping to detect central scotomas, and specialized color vision testing to assess cone function. These baseline measurements help establish the extent of visual impairment and guide treatment planning.
Advanced Imaging Techniques
Fundus Autofluorescence (FAF):
- Detects lipofuscin accumulation patterns
- A characteristic pattern of areas of increased and decreased signals on FAF imaging is seen in STGD
- Monitors disease progression over time
- Non-invasive and repeatable
Optical Coherence Tomography (OCT):
- OCT is a scanning device that works a little like an ultrasound
- Provides cross-sectional retinal images
- Quantifies retinal thickness changes
- Identifies specific layer involvement
Electroretinography (ERG):
- ERG measures electrical signals produced by the retina following flashes of light
- Assesses overall retinal function
- Differentiates between cone and rod involvement
- Provides objective functional measurements
Genetic Testing and Counseling
Benefits of Genetic Testing: Genetic testing provides a definitive diagnosis and determines specific inheritance patterns crucial for family planning. Advanced testing facilities, including those partnered with practices like Cannon EyeCare, can identify the specific genetic mutations causing a patient’s macular dystrophy, enabling eligibility assessment for targeted clinical trials and personalized treatment approaches.
Testing Process:
- Comprehensive genetic panel analysis using blood or saliva samples
- Professional genetic counseling to interpret results and implications
- Family member testing recommendations based on inheritance patterns
- Integration with clinical findings for a complete diagnostic picture
Current Treatment Approaches and Management Strategies
Evidence-Based Protective Interventions
Photoprotective Measures: Current clinical evidence supports specific photoprotective strategies to potentially slow disease progression. Patients should employ comprehensive ultraviolet and high-energy visible light protection through broad-spectrum sunglasses providing 100% UV filtration. Wide-brimmed hats offer additional ocular protection, particularly during outdoor activities.
Nutritional and Lifestyle Modifications: Rigorous adherence to vitamin A intake limitations represents a critical management component for ABCA4-related conditions. Patients should avoid supplemental vitamin A exceeding recommended daily allowances, as excessive intake may accelerate lipofuscin accumulation. Smoking cessation proves essential, given tobacco’s documented retinal toxicity and acceleration of photoreceptor degeneration.
Vision Rehabilitation and Adaptive Technologies
Low Vision Services: Comprehensive low vision rehabilitation encompasses multiple interventions tailored to individual functional needs. Optical magnification systems, including hand-held and stand magnifiers, electronic video magnification devices, and telescopic systems, can significantly enhance remaining visual function. Training in eccentric viewing techniques helps patients optimize their peripheral vision for central tasks.
Technology Integration: Modern assistive technologies offer unprecedented opportunities for maintaining independence. Screen reading software, voice recognition systems, and smartphone applications specifically designed for low vision users can facilitate continued participation in educational, professional, and recreational activities.
Emerging Therapies
Pharmacological Approaches: Clinical trials are investigating several promising medications:
- Gildeuretinol (ALK-001): This deuterated form of vitamin A has received Breakthrough Therapy Designation from the FDA. Recent TEASE-3 trial results (January 2025) showed complete prevention of disease progression in early-stage Stargardt patients for up to 6 years, representing the first treatment to halt disease progression
- Visual Cycle Modulators: Compounds that slow toxic byproduct formation by targeting specific enzymes in the visual cycle
- Complement Inhibitors: Targeting inflammation pathways, with some agents approved for geographic atrophy now being tested in Stargardt disease
- Neuroprotective Agents: Preserving remaining retinal cells through various protective mechanisms
Breakthrough Research and Clinical Trials
Gene Therapy Developments
Adeno-Associated Virus (AAV) Vectors: Gene therapy represents a promising prophylactic treatment of monogenic retinal disorders, including Stargardt disease. However, challenges exist due to the large size of therapeutic genes like ABCA4.
Dual-AAV Strategies:
- Splitting large genes across multiple vectors
- Recombining therapeutic sequences in target cells
- Showing promise in animal models
- Moving toward human trials
Non-Viral Approaches: Treatment with ECO/pRHO-ABCA4 resulted in a significant reduction (35%) of A2E accumulation in Abca4−/− mice 6 months after a single injection, demonstrating potential for alternative delivery methods.
Stem Cell Therapy
Current Clinical Trials: Multiple ongoing studies are evaluating promising therapies:
- Gildeuretinol (ALK-001): Following breakthrough TEASE-3 results showing complete prevention of progression in early-stage patients, the TEASE-2 trial with 80 participants is expected to report results in 2025
- ASP7317 Stem Cell Therapy: Phase Ib trial is actively enrolling patients with improved hESC-RPE cells, with results expected in late 2025
- Gene Therapy Approaches: Multiple viral and non-viral gene delivery systems are being tested for ABCA4 gene replacement
- RNA Editing: ACDN-01 by Ascidian Therapeutics received Fast Track designation for RNA exon editing therapy targeting ABCA4 mutations
Results and Progress:
- Stem cell therapy using human pluripotent stem cell-derived retinal pigment epithelium cells demonstrated long-term safety three years after implantation and visual acuity improvements in the first two years after initiation of therapy
- Ongoing Phase 2 trials with improved cell preparations
- Focus on safety and preliminary efficacy data
Future Directions:
- Induced pluripotent stem cells (iPSCs) for personalized therapy
- Tissue engineering approaches
- Combination therapies with gene therapy
Recent Scientific Studies
Study 1: Machine Learning Applications (2024) Recent advancements in machine learning (ML) and deep learning show considerable promise in automating these processes for disease monitoring and phenotyping. This research from 2024 demonstrates how AI can:
- Automate retinal image analysis
- Standardize disease progression measurements
- Reduce clinician workload
- Improve clinical trial efficiency
Study 2: TEASE-3 Clinical Trial Results (January 2025) Alkeus Pharmaceuticals announced breakthrough results from the TEASE-3 study, showing that gildeuretinol (ALK-001) completely prevented disease progression in early-stage Stargardt patients for up to 6 years. Three teenagers enrolled in the study remained symptom-free and progression-free, with one patient stable for 2 years and two patients for 6 years – marking the first time any treatment has halted Stargardt disease progression.
Study 3: Advanced Stem Cell Therapy Trials (2024) The Phase Ib 7317-CL-0003 trial by Astellas is actively enrolling patients with improved hESC-RPE cells (ASP7317) for both geographic atrophy and Stargardt macular dystrophy, with results expected in late 2025. This represents the next generation of stem cell therapies following successful safety data from earlier trials.
Living with Macular Dystrophy
Emotional and Psychological Support
Coping Strategies: Receiving a macular dystrophy diagnosis, especially during childhood or young adulthood, can be emotionally challenging. Always maintain hope and adaptation—the human spirit and body are so amazing and able to adapt. I have patients who are athletes in Paralympics and Paralympians and patients with Stargardt who have authored books and climbed mountains.
Professional Support:
- Counseling for adjustment to vision loss
- Support groups for patients and families
- Peer mentoring programs
- Educational advocacy services
Family Impact:
- Genetic counseling for family planning
- Sibling and parent support resources
- Educational system navigation
- Workplace accommodation guidance
Educational and Career Considerations
Academic Accommodations:
- Extended time for examinations
- Large print or digital materials
- Assistive technology training
- Lighting modifications
Career Planning:
- Vocational rehabilitation services
- Job accommodation assessments
- Skills training programs
- Career counseling focused on abilities
Technology and Independence
Assistive Technologies:
- Smartphone accessibility features
- Computer screen magnification
- Audio book services
- GPS navigation systems
Home Modifications:
- Improved lighting systems
- High-contrast marking techniques
- Organization strategies
- Safety modifications
Prevention and Management Strategies
Primary Prevention
While macular dystrophy cannot be prevented due to its genetic nature, understanding family history enables:
- Early screening for at-risk individuals
- Genetic counseling for family planning
- Proactive vision monitoring
- Timely intervention when symptoms appear
Secondary Prevention (Slowing Macular Dystrophy Progression)
Managing Disease Progression: Research has shown that certain lifestyle modifications can help slow the progression of macular dystrophy symptoms in some patients. While these measures cannot cure the underlying genetic condition, they may help preserve remaining vision for longer periods.
Regular Monitoring and Follow-up Care
Ongoing Assessment: Regular comprehensive eye examinations remain essential for monitoring disease progression and adjusting management strategies. Eye care practices that specialize in inherited retinal diseases, such as Cannon EyeCare, provide coordinated care including advanced imaging, functional testing, and genetic counseling services. Annual examinations help track progression patterns and identify candidates for emerging therapies.
Tertiary Prevention
Maintaining Quality of Life:
- Low vision rehabilitation
- Adaptive skills training
- Social support maintenance
- Independence preservation strategies
Future Outlook and Research Directions
Promising Therapeutic Avenues
Gene Editing Technologies:
- CRISPR/Cas9 applications for genetic correction
- Base editing for precise mutations
- Prime editing for complex genetic changes
- In vivo gene editing development
Combination Therapies:
- Gene therapy with neuroprotection
- Stem cells with pharmacological support
- Multi-target therapeutic approaches
- Personalized medicine strategies
Advanced Delivery Systems:
- Improved viral vectors
- Nanoparticle delivery systems
- Sustained release formulations
- Targeted cellular delivery
Clinical Trial Opportunities
Patients interested in clinical trial participation should:
- Discuss options with their eye care provider
- Consider travel requirements for specialized centers
- Understand trial phases and expectations
- Ask your eye care specialist if you’d be a good fit for a clinical trial. This can help you get access to new, experimental treatments.
References and Additional Resources
Key Scientific Publications and Medical Sources
1. National Eye Institute – Stargardt Disease Overview
Source: National Institutes of Health
https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/stargardt-disease
Comprehensive patient education resource from the NIH providing current information on Stargardt disease symptoms, diagnosis, and emerging treatments. This government source offers evidence-based medical guidance and updates on clinical trial opportunities.
2. Macular Dystrophies: Clinical and Imaging Features, Molecular Genetics and Therapeutic Options
Published in: British Journal of Ophthalmology – PMC7147237
https://pmc.ncbi.nlm.nih.gov/articles/PMC7147237/
Peer-reviewed medical literature review covering the most common subtypes of macular dystrophy, including detailed clinical features, retinal imaging advances, molecular genetics, and ongoing clinical trials for gene therapy, cellular therapy, and pharmacological treatments.
3. Stargardt’s Disease: Molecular Pathogenesis and Current Therapeutic Landscape (2024)
Published in: Current Ophthalmology Reports – PMC12295471
https://pmc.ncbi.nlm.nih.gov/articles/PMC12295471/
Recent comprehensive analysis of ABCA4-related Stargardt disease pathophysiology and current therapeutic developments, including detailed coverage of breakthrough clinical trials and emerging treatment modalities as of 2024-2025.
Professional Publication Summary
This comprehensive clinical review examines macular dystrophy—a collection of inherited retinal disorders characterized by progressive macular degeneration in children and young adults. The analysis synthesizes current understanding of pathophysiology, diagnostic approaches, and therapeutic developments, with particular emphasis on breakthrough clinical trial results from 2025.
Key findings include significant advances in genetic characterization, with over 1,000 ABCA4 variants identified in Stargardt disease alone. Diagnostic capabilities have improved through multimodal imaging techniques, while therapeutic development has reached unprecedented milestones. The TEASE-3 clinical trial demonstrated complete disease progression arrest in early-stage patients treated with gildeuretinol, representing the first therapeutic success in halting hereditary macular degeneration.
Future research directions encompass gene therapy approaches, cellular replacement strategies, and combination therapeutic regimens. The evolving therapeutic landscape offers renewed hope for patients and families affected by these previously intractable conditions, emphasizing the critical importance of early diagnosis and intervention.
Immediate Action Steps:
- Seek Comprehensive Evaluation: If you suspect macular dystrophy symptoms, schedule a complete eye examination with a retinal specialist or comprehensive eye care provider such as Cannon EyeCare
- Consider Genetic Testing: Confirm diagnosis and understand inheritance patterns for family planning
- Implement Protective Measures: Begin UV protection, avoid smoking, and optimize nutrition
- Connect with Support Services: Access low vision rehabilitation and emotional support resources
- Stay Informed: Follow research developments and clinical trial opportunities
Looking Forward
The landscape of macular dystrophy treatment continues to evolve rapidly. While multiple potential interventions have shown promise in attenuating disease progression, further exploration is necessary to demonstrate treatment safety and efficacy. Patients diagnosed today have concrete reasons for optimism as research advances accelerate and new therapeutic options move through clinical development.
Take Action Today:
- Schedule an eye exam if you notice vision changes
- Discuss family history with your eye doctor
- Research clinical trial opportunities in your area
- Connect with patient advocacy organizations
- Consider genetic counseling for family planning
Early diagnosis, comprehensive care, and proactive management strategies significantly impact long-term outcomes. By working with experienced eye care providers like Cannon EyeCare, utilizing available resources, and staying informed about research developments, individuals with macular dystrophy can maintain active, fulfilling lives while preparing for emerging treatment opportunities.
Emergency Warning Signs – Seek Immediate Care:
- Sudden vision loss or dramatic vision changes
- New onset of flashing lights or floaters
- Curtain-like vision loss
- Severe eye pain with vision changes
Medical Disclaimer: This article is for educational purposes only and should not replace professional medical consultation. Individual symptoms, progression, and treatment responses may vary significantly.
FAQs
-
Macular dystrophy is a genetic eye disorder causing progressive loss of central vision due to damage in the macula, the center of the retina responsible for detailed sight.